Antibacterial Effect of Topically Administered Tranexamic Acid in Large Joint Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Microdilution Technique for the Determination of MIC and MBC of Tranexamic Acid
2.3. Kinetic Growth Assay
2.4. Statistics
3. Results
3.1. Determination of the Antiproliferative Effect of Different TXA Concentrations with the Microdilution Technique
3.2. Kinetic Growth Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akonjom, M.; Battenberg, A.; Beverland, D.; Choi, J.-H.; Fillingham, Y.; Gallagher, N.; Han, S.B.; Jang, W.Y.; Jiranek, W.; Manrique, J.; et al. General Assembly, Prevention, Blood Conservation: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S147–S155. [Google Scholar] [CrossRef]
- Viola, J.; Gomez, M.M.; Restrepo, C.; Maltenfort, M.G.; Parvizi, J. Preoperative Anemia Increases Postoperative Complications and Mortality Following Total Joint Arthroplasty. J. Arthroplast. 2015, 30, 846–848. [Google Scholar] [CrossRef]
- Lu, M.; Sing, D.C.; Kuo, A.C.; Hansen, E.N. Preoperative Anemia Independently Predicts 30-Day Complications after Aseptic and Septic Revision Total Joint Arthroplasty. J. Arthroplast. 2017, 32, S197–S201. [Google Scholar] [CrossRef] [PubMed]
- Maempel, J.F.; Wickramasinghe, N.R.; Clement, N.D.; Brenkel, I.J.; Walmsley, P.J. The Pre-Operative Levels of Haemoglobin in the Blood Can Be Used to Predict the Risk of Allogenic Blood Transfusion after Total Knee Arthroplasty. Bone Jt. J. 2016, 98-B, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, C.; Agaoglu, L.; Karakas, Z.; Gurel, N.; Yalcin, I. The Effect of Iron Deficiency Anemia on the Function of the Immune System. Hematol. J. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.; Homering, M.; Holberg, G.; Berkowitz, S. Allogeneic Blood Transfusions and Postoperative Infections after Total Hip or Knee Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2014, 96, 272–278. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Chen, W.; Liu, S.; Zhang, Q.; Zhang, Y. Risk Factors for Periprosthetic Joint Infection after Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2015, 89, 82–89. [Google Scholar] [CrossRef]
- Barrachina, B.; Lopez-Picado, A.; Remon, M.; Fondarella, A.; Iriarte, I.; Bastida, R.; Rodríguez-Gascón, A.; Achaerandio, M.A.; Iturricastillo, M.C.; Aizpuru, F.; et al. Tranexamic Acid Compared with Placebo for Reducing Total Blood Loss in Hip Replacement Surgery: A Randomized Clinical Trial. Anesth. Analg. 2016, 122, 986–995. [Google Scholar] [CrossRef]
- Yang, Z.-G.; Chen, W.-P.; Wu, L.-D. Effectiveness and Safety of Tranexamic Acid in Reducing Blood Loss in Total Knee Arthroplasty: A Meta-Analysis. J. Bone Jt. Surg. Am. Vol. 2012, 94, 1153–1159. [Google Scholar] [CrossRef]
- Fillingham, Y.A.; Ramkumar, D.B.; Jevsevar, D.S.; Yates, A.J.; Shores, P.; Mullen, K.; Bini, S.A.; Clarke, H.D.; Schemitsch, E.; Johnson, R.L.; et al. The Efficacy of Tranexamic Acid in Total Hip Arthroplasty: A Network Meta-Analysis. J. Arthroplast. 2018, 33, 3083–3089.e4. [Google Scholar] [CrossRef]
- Fillingham, Y.A.; Ramkumar, D.B.; Jevsevar, D.S.; Yates, A.J.; Shores, P.; Mullen, K.; Bini, S.A.; Clarke, H.D.; Schemitsch, E.; Johnson, R.L.; et al. The Efficacy of Tranexamic Acid in Total Knee Arthroplasty: A Network Meta-Analysis. J. Arthroplast. 2018, 33, 3090–3098.e1. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Evans, H.M.K.; Mahomed, S.R.; Mahomed, N.N. Tranexamic Acid and the Reduction of Blood Loss in Total Knee and Hip Arthroplasty: A Meta-Analysis. BMC Res. Notes 2013, 6, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukeik, M.; Alshryda, S.; Haddad, F.S.; Mason, J.M. Systematic Review and Meta-Analysis of the Use of Tranexamic Acid in Total Hip Replacement. J. Bone Jt. Surg. Br. Vol. 2011, 93, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukeik, M.; Alshryda, S.; Powell, J.; Haddad, F.S. The Effect of Tranexamic Acid on Wound Complications in Primary Total Hip Arthroplasty: A Meta-Analysis. Surgeon 2020, 18, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, H.; Klement, M.R.; Hammad, M.; Inoue, D.; Xu, C.; Goswami, K.; Parvizi, J. Tranexamic Acid Is Associated with Reduced Periprosthetic Joint Infection after Primary Total Joint Arthroplasty. J. Arthroplast. 2020, 35, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Björlin, G.; Nilsson, I.M. The Effect of Antifibrinolytic Agents on Wound Healing. Int. J. Oral Maxillofac. Surg. 1988, 17, 275–276. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, M.; Raina, P.; Singh, S.; Ahmad, S.; Imran, S.; Sharma, R.; Malhotra, S. Post-Surgical Wound Care in Orthopedics: Role of Tranexamic Acid. J. Evol. Med. Dent. Sci. 2015, 4, 5716–5720. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, X.-M.; Yang, L.-J.; Wu, P.-L. Tranexamic Acid Accelerates Skin Barrier Recovery and Upregulates Occludin in Damaged Skin. Int. J. Derm. 2014, 53, 959–965. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Ribeiro, A.L.C.; Costa, B.R.C.; Vago, J.P.; Lima, K.M.; Carneiro, F.S.; Ortiz, M.M.O.; Lima, G.L.N.; Carmo, A.A.F.; Rocha, R.M.; et al. Plasmin and Plasminogen Induce Macrophage Reprogramming and Regulate Key Steps of Inflammation Resolution via Annexin A1. Blood 2017, 129, 2896–2907. [Google Scholar] [CrossRef] [Green Version]
- Borg, R.J.; Samson, A.L.; Au, A.E.-L.; Scholzen, A.; Fuchsberger, M.; Kong, Y.Y.; Freeman, R.; Mifsud, N.A.; Plebanski, M.; Medcalf, R.L. Dendritic Cell-Mediated Phagocytosis but Not Immune Activation Is Enhanced by Plasmin. PLoS ONE 2015, 10, e0131216. [Google Scholar] [CrossRef] [Green Version]
- Ausen, K.; Pleym, H.; Liu, J.; Hegstad, S.; Nordgård, H.B.; Pavlovic, I.; Spigset, O. Serum Concentrations and Pharmacokinetics of Tranexamic Acid after Two Means of Topical Administration in Massive Weight Loss Skin-Reducing Surgery. Plast. Reconstr. Surg. 2019, 143, 1169–1178. [Google Scholar] [CrossRef]
- Kim, Y.T.; Kang, M.W.; Lee, J.K.; Lee, Y.M.; Kim, J.I. Combined Use of Topical Intraarticular Tranexamic Acid and Rivaroxaban in Total Knee Arthroplasty Safely Reduces Blood Loss, Transfusion Rates, and Wound Complications without Increasing the Risk of Thrombosis. BMC Musculoskelet. Disord. 2018, 19, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitek, P.; Wysocka-Wycisk, A.; Kępski, F.; Król, D.; Bursig, H.; Dyląg, S. PRP-Fibrinogen Gel-like Chondrocyte Carrier Stabilized by TXA-Preliminary Study. Cell Tissue Bank 2013, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.; McCall, K.; Smith, I.D.M.; Blyth, M.; Kitson, S.M.; Crowe, L.A.N.; Leach, W.J.; Rooney, B.P.; Spencer, S.J.; Mullen, M.; et al. Tranexamic Acid Toxicity in Human Periarticular Tissues. Bone Jt. Res. 2019, 8, 11–18. [Google Scholar] [CrossRef]
- Eikebrokk, T.A.; Vassmyr, B.S.; Ausen, K.; Gravastrand, C.; Spigset, O.; Pukstad, B. Cytotoxicity and Effect on Wound Re-epithelialization after Topical Administration of Tranexamic Acid. BJS Open 2019, 3, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Furst, W.; Banerjee, A.; Redl, H. Comparison of Structure, Strength and Cytocompatibility of a Fibrin Matrix Supplemented Either with Tranexamic Acid or Aprotinin. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 109–114. [Google Scholar] [CrossRef]
- Benjumea, A.; Díaz-Navarro, M.; Hafian, R.; Sánchez-Somolinos, M.; Vaquero, J.; Chana, F.; Muñoz, P.; Guembe, M. Effect of Tranexamic Acid against Staphylococcus Spp. and Cutibacterium Acnes Associated with Peri-Implant Infection: Results from an In Vitro Study. Microbiol. Spectr. 2022, 10, e01612-21. [Google Scholar] [CrossRef]
- Benjumea, A.; Díaz-Navarro, M.; Hafian, R.; Cercenado, E.; Sánchez-Somolinos, M.; Vaquero, J.; Chana, F.; Muñoz, P.; Guembe, M. Tranexamic Acid in Combination with Vancomycin or Gentamicin Has a Synergistic Effect against Staphylococci. Front. Microbiol. 2022, 13, 935646. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Li, J.; Huang, B.; Jiang, Z.; Pan, Y.; He, T.; Hu, Y.; Wang, L. Tranexamic Acid Protects against Implant-Associated Infection by Reducing Biofilm Formation. Sci. Rep. 2022, 12, 4840. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Brewster, J.D. A Simple Micro-Growth Assay for Enumerating Bacteria. J. Microbiol. Methods 2003, 53, 77–86. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van’t Riet, K. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Frisch, N.B.; Wessell, N.M.; Charters, M.A.; Yu, S.; Jeffries, J.J.; Silverton, C.D. Predictors and Complications of Blood Transfusion in Total Hip and Knee Arthroplasty. J. Arthroplast. 2014, 29, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.S.; Sojka, J.H.; Mayerson, J.L.; Glassman, A.H.; Scharschmidt, T.J. Perioperative Allogeneic Red Blood-Cell Transfusion Associated with Surgical Site Infection after Total Hip and Knee Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2018, 100, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Mur, I.; Ribera, A.; Soriano, A.; Rodríguez-Pardo, D.; Sorlí, L.; Cobo, J.; Fernández-Sampedro, M.; del Toro, M.D.; Guío, L.; et al. The Different Microbial Etiology of Prosthetic Joint Infections According to Route of Acquisition and Time after Prosthesis Implantation, Including the Role of Multidrug-Resistant Organisms. J. Clin. Med. 2019, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute [CLSI] Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 2018. Available online: https://clsi.org/standards/products/microbiology/documents/m07 (accessed on 19 February 2023).
- Theophel, K.; Schacht, V.J.; Schlüter, M.; Schnell, S.; Stingu, C.-S.; Schaumann, R.; Bunge, M. The Importance of Growth Kinetic Analysis in Determining Bacterial Susceptibility against Antibiotics and Silver Nanoparticles. Front. Microbiol. 2014, 5, 544. [Google Scholar] [CrossRef]
- Ahlberg, A.; Eriksson, O.; Kjellman, H. Diffusion of Tranexamic Acid to the Joint. Acta Orthop. Scand. 1976, 47, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, I.M. Clinical Pharmacology of Aminocaproic and Tranexamic Acids. J. Clin. Pathol. Suppl. (R. Coll. Pathol.) 1980, 14, 41–47. [Google Scholar] [CrossRef] [Green Version]
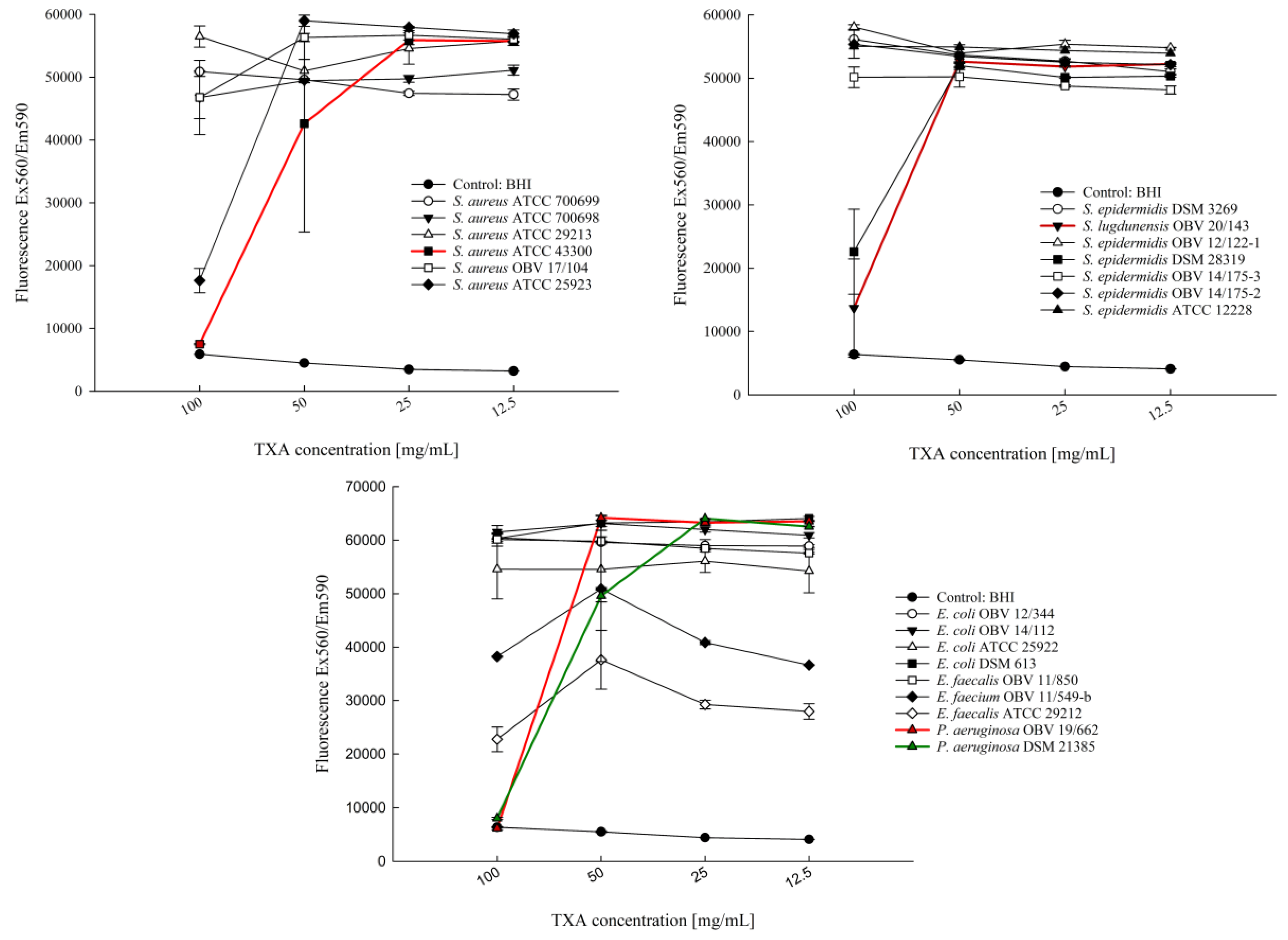
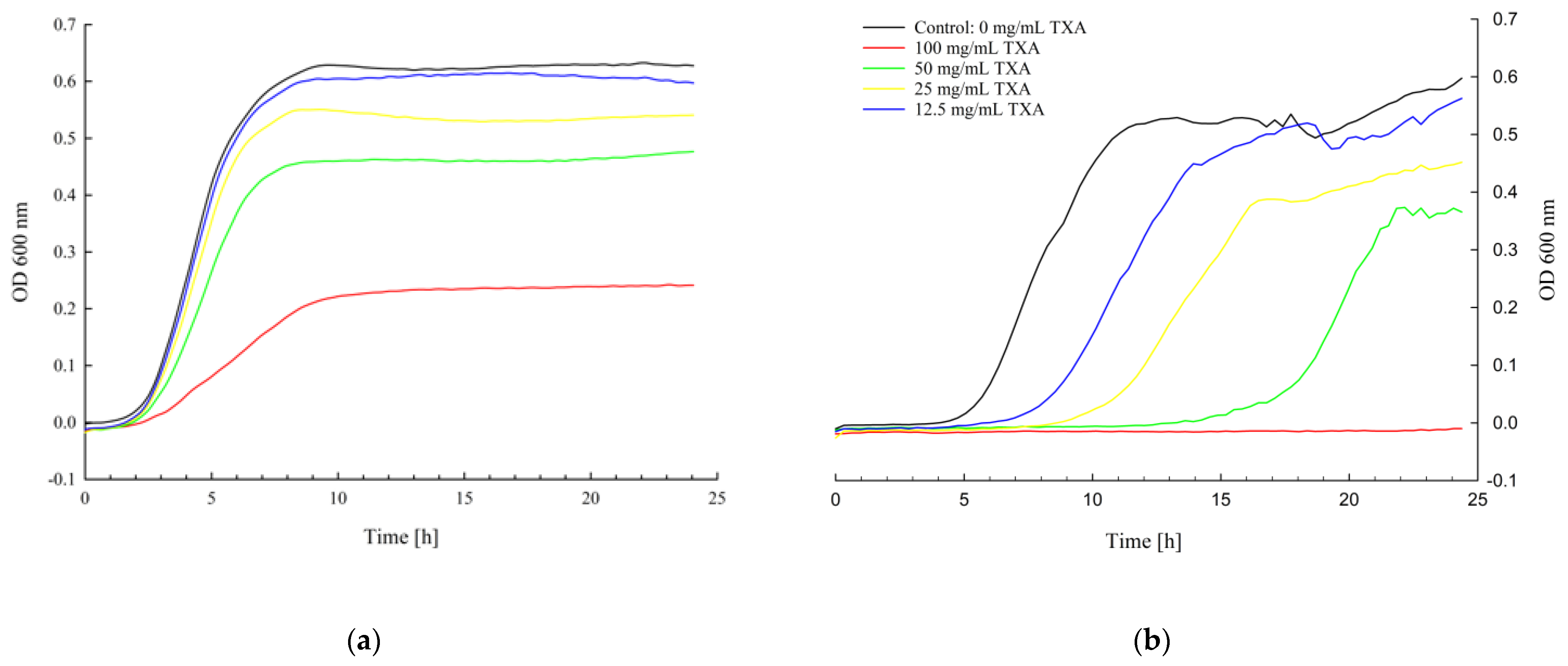


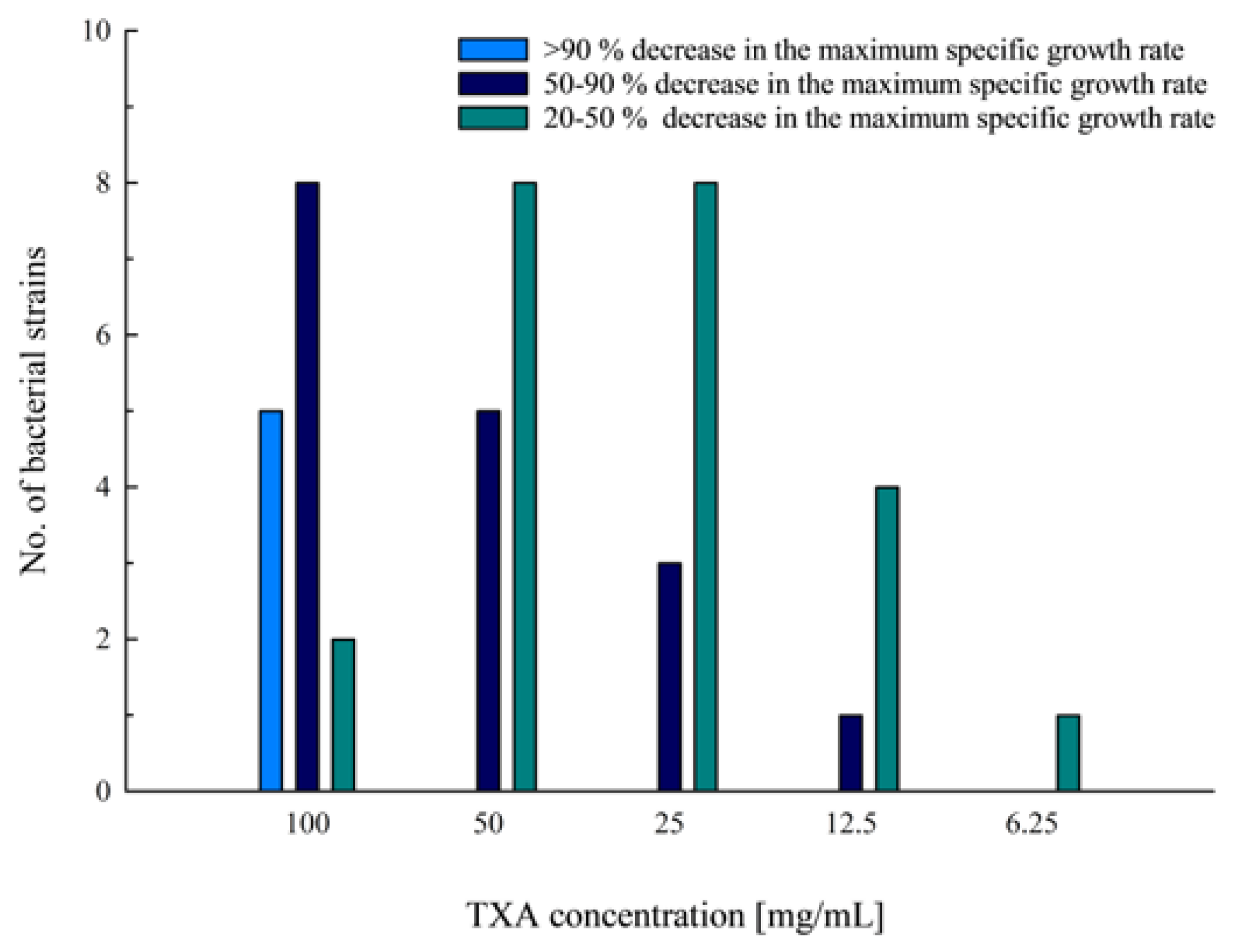
| Bacteria | Strain | Strain Details |
|---|---|---|
| Staphylococcus aureus | ATCC 25923 | Clinical isolate |
| Staphylococcus aureus | ATCC 29213 | Clinical isolate; wound |
| Staphylococcus aureus (MRSA) | ATCC 43300 | Clinical isolate; resistant to methicillin and oxacillin |
| Staphylococcus aureus (MRSA) | ATCC 700698 | Clinical isolate; sputum from lung cancer patient with MRSA pneumonia; resistant to methicillin |
| Staphylococcus aureus (MRSA) | ATCC 700699 | Clinical isolate; from pus and debrided tissue at surgical incision in sternum of an infant; resistant to methicillin and oxacillin; reduced vancomycin susceptibility |
| Staphylococcus aureus (MRSA) | OBV 17/104 | Clinical isolate; PJI; resistant to methicillin |
| Staphylococcus epidermidis | DSM 28319 | Clinical isolate; catheter sepsis |
| Staphylococcus epidermidis | DSM 3269 | Clinical isolate; infected catheter tip; resistant to aztreonam |
| Staphylococcus epidermidis | ATCC 12228 | Non-infection associated strain |
| Staphylococcus epidermidis | OBV 14/175-2 | Clinical isolate; PJI |
| Staphylococcus epidermidis | OBV 14/175-3 | Clinical isolate; PJI; resistant to erythromycin, clindamycin, and oxacillin |
| Staphylococcus epidermidis | OBV 12/122 | Clinical isolate; PJI |
| Staphylococcus lugdunensis | OBV 20/143 | Clinical isolate; PJI |
| Pseudomonas aeruginosa | DSM 21385 | Human pathogen |
| Pseudomonas aeruginosa | OBV 19/662 | Clinical isolate; PJI; resistant to ciprofloxacin |
| Enterococcus faecalis | ATCC 29212 | Clinical isolate; uroinfection |
| Enterococcus faecalis | OBV 11/850 | Clinical isolate; PJI |
| Enterococcus faecium | OBV 11/549-b | Clinical isolate; PJI |
| Escherichia coli | ATCC 25922 | Clinical isolate |
| Escherichia coli | DSM 613 | B strain |
| Escherichia coli | OBV 14/112 | Clinical isolate; PJI; resistant to quinolones |
| Escherichia coli | OBV 12/344 | Clinical isolate; PJI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slokar, U.; Kovač, S.; Cör, A.; Šuster, K. Antibacterial Effect of Topically Administered Tranexamic Acid in Large Joint Arthroplasty. Appl. Sci. 2023, 13, 9050. https://doi.org/10.3390/app13169050
Slokar U, Kovač S, Cör A, Šuster K. Antibacterial Effect of Topically Administered Tranexamic Acid in Large Joint Arthroplasty. Applied Sciences. 2023; 13(16):9050. https://doi.org/10.3390/app13169050
Chicago/Turabian StyleSlokar, Urban, Simon Kovač, Andrej Cör, and Katja Šuster. 2023. "Antibacterial Effect of Topically Administered Tranexamic Acid in Large Joint Arthroplasty" Applied Sciences 13, no. 16: 9050. https://doi.org/10.3390/app13169050







